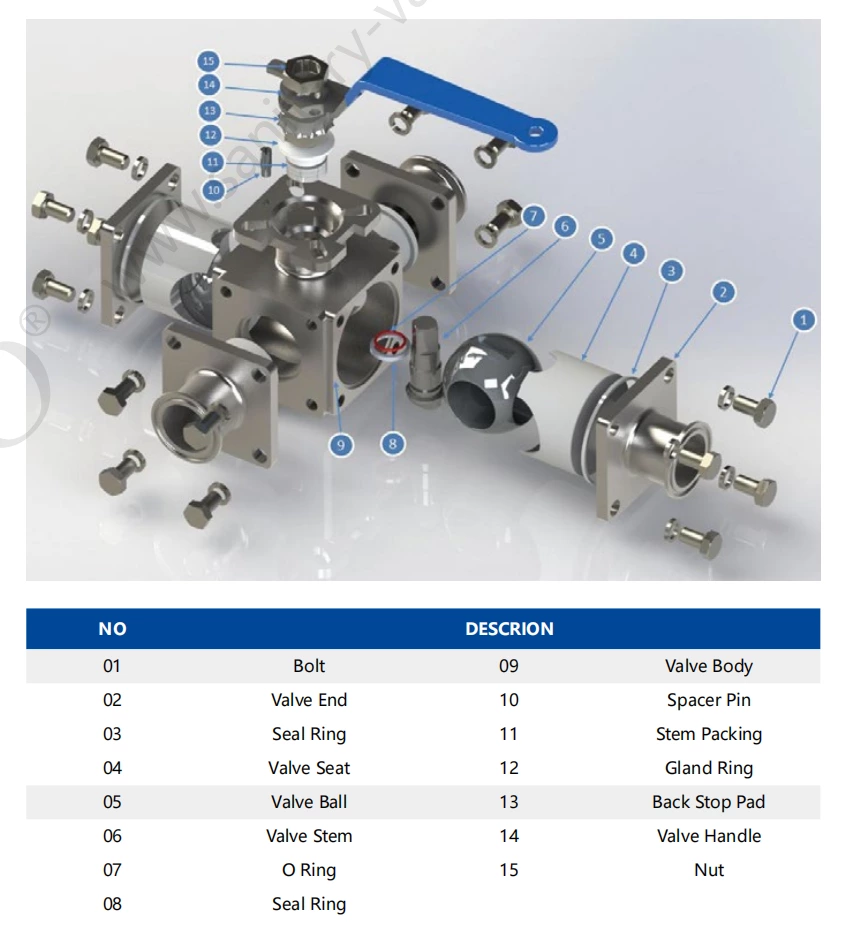
Sanitary Stainless Steel Encapsulated Clamped Four Ways Ball Valve
- Place of Origin:
- Wenzhou, China
- Packing:
- Suitable Packing for Sea/Air Transport
- Quantity:
- 10000 PCS Per Year
- Payment:
- T/T, L/C, Western Union
- Medium:
- Water, Oil
- Material:
- SS316L
- Connection:
- Clamped
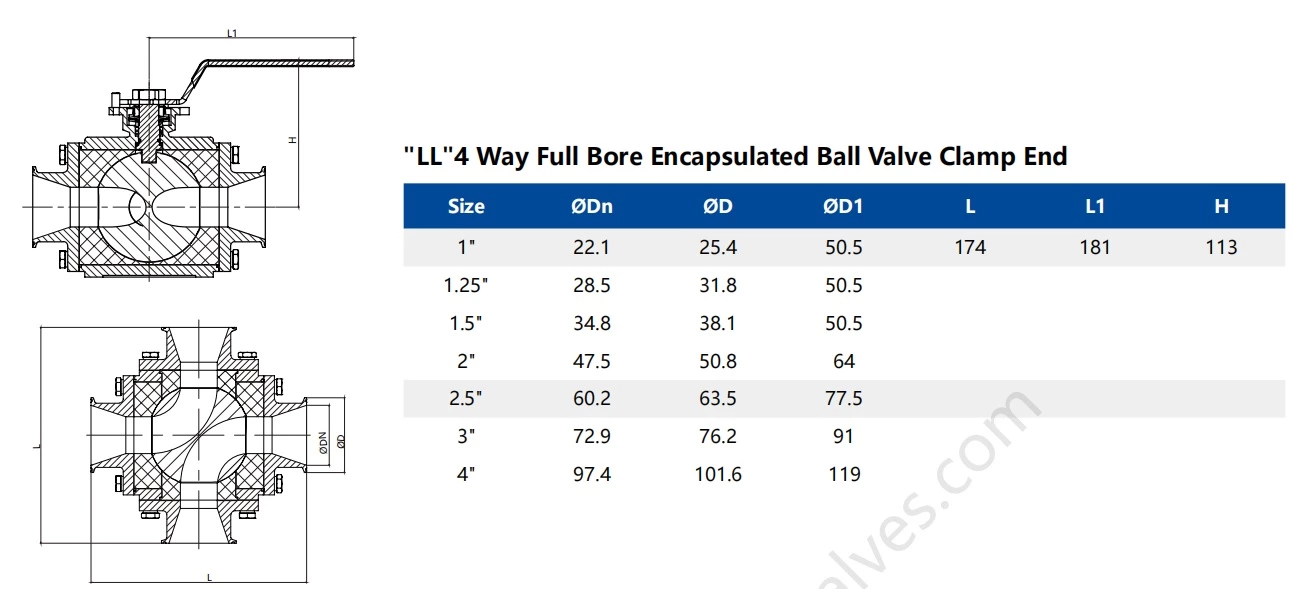
- Specification:
- 1/2"-4"
- Pressure:
- 0-10Bar
- Temperature:
- -20°~120°
- Gasket:
- PTFE
- Shape:
- Square
- Type:
- LType/T Type/LL Type
Sanitary Stainless Steel Encapsulated Clamp Four Ways Ball Valve
- Sanitary encapsulated 4-ways ball valve also can be called sanitary cavity filled four way ball valve. It can be applied to the pipeline system in the industries of food, wine, brewery, oil refining, cosmetics, pharmaceuticals and chemicals that requires non-retention and simultaneous switch of flow directions of two media.
- Features of cavity filled four way valves:
• Small fluid resistance: The cross section of the ball, valve body and connecting pipe are all equal, and the ball channel is arc design, when the medium passes through the valve, the fluid resistance is small.
• Good sealing performance: The valve seat is made of PTFE material with a certain elastic deformation and high strength to reach the good sealing performance.That ensures the stability of the valve.
• Long service life: Overall material of the valve is sanitary authentic stainless steel and the material of the valve seat is PTFE,which can reach the good effect of corrosion resistance and extend the service life of the valve.
• Non-retention design: high-quality PTFE encapsulated structure is selected for sealing. The PTFE valve seat is in close contact with the ball and the valve body, so as to achieve the function of non-retention.
Product Description
| Valve Body Material: | SS316L |
| Seal Material: | PTFE |
| Max working pressure: | 10Bar |
| Max. Working Temperature: | 120 degree C |
| Availably size: | 1"-6", DN25-DN150 |
| Availably connection: | Clamped |
| Availably standard: | 3A/DIN/SMS/RJT/ISO/IDF |
| Operated: | Manual/Pneumatic/Electrical |
| Certificate: | ISO, PED/97/23/EC, FDA.177.2600 |
Product Parameter
Product Pictures


















